Bispecific Antibodies Market Challenges and Risk Profile
Report Overview
The Global Bispecific Antibodies Market size is expected to be worth around USD 192.6 Billion by 2033 from USD 8.0 Billion in 2023, growing at a CAGR of 37.5% during the forecast period from 2024 to 2033.
The Bispecific Antibodies Market is entering a phase of manufacturing optimization and scale-up in 2025, paving the way for broader clinical accessibility. Historically known for complex production and low yield, bispecific antibodies are now benefiting from innovations like single-chain formats, modular plug-and-play platforms, and cell-free synthesis techniques. Leading CDMOs are investing in specialized bioreactors to meet demand for BsAb clinical and commercial batches.
Additionally, regulatory frameworks are evolving to accommodate fast-track approval pathways for BsAbs in high-unmet-need areas. With scalable manufacturing and clearer regulatory routes, the entry barriers for smaller biotech firms are lowering. These shifts are enabling a more competitive and diverse pipeline, ultimately reducing costs and increasing patient access. As a result, the market is becoming not just innovative but also commercially viable on a global scale.
Click here for more information: https://market.us/report/bispecific-antibodies-market/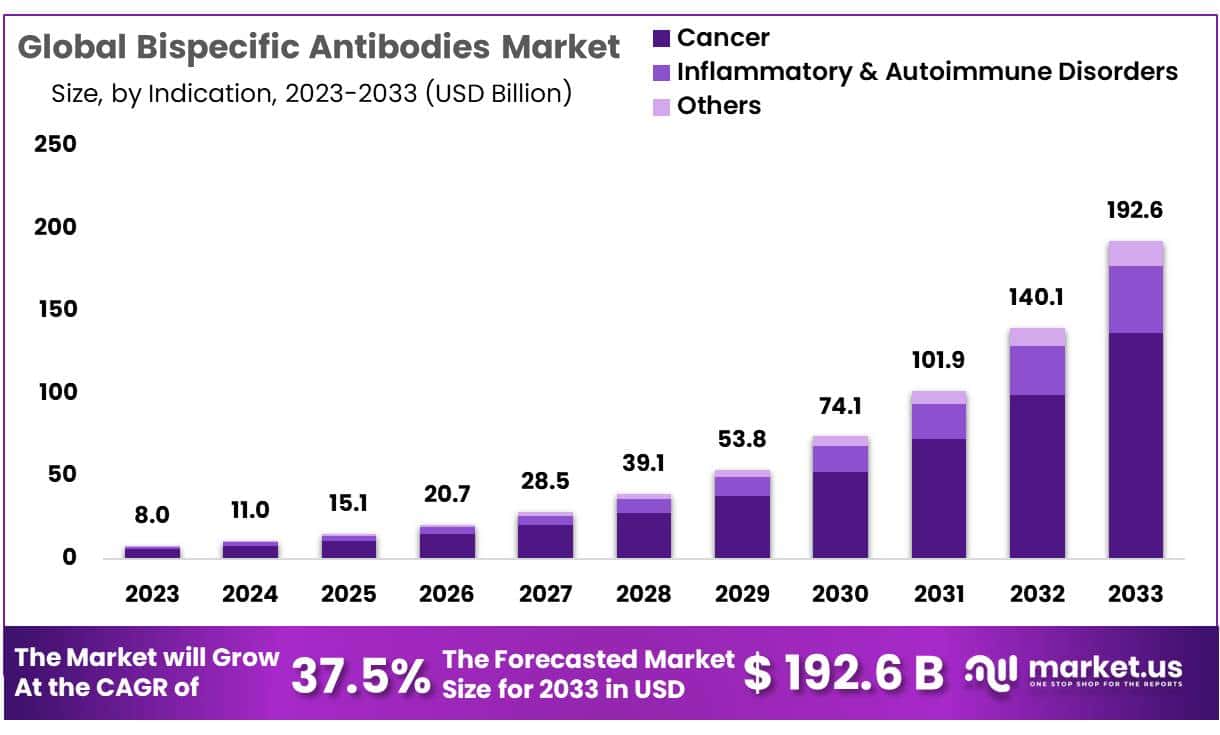
Key Market Segments
Indication
- Cancer
- Inflammatory & Autoimmune Disorders
- Others
End-User
- Hospitals
- Speciality Clinics
- Homecare
Market Key Players
- Amgen
- F. Hoffmann-La Roche Ltd
- Genentech
- Akeso, Inc.
- Janssen
- Taisho Pharmaceutical
- MacroGenics, Inc
- CELGENE CORPORATION
- Immunocore
- Sanofi
- AstraZeneca
Get a Sample Copy of the Report to Know More: https://market.us/report/bispecific-antibodies-market/request-sample/
Emerging Trends
A key trend in 2025 is the industrialization of bispecific antibody manufacturing. Companies are moving toward unified bispecific platforms that streamline expression, purification, and QC processes. Flexible CMC strategies now accommodate a variety of BsAb structures within a single process flow.
Additionally, low-immunogenic scaffold formats are being standardized, allowing faster scale-up with fewer safety hurdles. These advances are removing technical roadblocks and setting the stage for rapid expansion of the clinical pipeline.
Use Cases
A global CDMO partnered with a biotech firm developing a bispecific antibody for non-small cell lung cancer. By using a platform-based production model, they reduced time-to-batch release by 40%. Automation tools were used for glycosylation profiling and aggregate detection, maintaining consistency across multiple BsAb formats.
This enabled the biotech to launch simultaneous Phase II trials in the U.S., EU, and APAC. The case shows how scalable infrastructure and modular workflows can accelerate go-to-market timelines for BsAb developers.
Contact us on
Market.us (Powered By Prudour Pvt. Ltd.)
Email: inquiry@market.us
Address: 420 Lexington Avenue, Suite 300,
New York City, NY 10170, United States
Tel: +1 718 618 4351
Comments
Post a Comment